The Science of Rare Isotopes
From the Cosmological Origin of the Elements to Making Rare Isotopes for Use on Earth
Alexandra Gade
Professor of Physics
Scientific Director, Facility for Rare Isotope Beams
Michigan State University
Sponsored by a PSW Science member who wishes to remain anonymous
About the Lecture
There are approximately 300 stable atomic nuclei and 3,000 known unstable (rare) isotopes that are radioactive and that eventually decay into their stable cousins. Estimates are that over 7,000 different isotopes can be forged that are bound by the nuclear force. It is now recognized that the properties of many yet undiscovered rare isotopes hold the key to understanding how to develop a comprehensive and predictive model of atomic nuclei, to accurately model a variety of astrophysical environments, and to understand the origin and history of elements in the Universe. Some of these isotopes also offer the possibility to study nature’s underlying fundamental symmetries and to explore new applications of rare isotopes. This presentation will give a glimpse of the opportunities that arise at the Facility for Rare Isotope Beams (FRIB) that started operations at Michigan State University in 2022.
Selected Reading & Media References
https://www.energy.gov/science/np/articles/facility-rare-isotope-beams-after-one-year-operation
https://www.energy.gov/science/np/articles/facility-rare-isotope-beams-observes-five-never-seen-isotopes
https://frib.msu.edu/science
https://frib.msu.edu/science/structure
https://frib.msu.edu/science/astrophysics
https://pubs.aip.org/physicstoday/article-pdf/61/11/40/8318835/40_1_online.pdf
About the Speaker

Alexandra Gade is Professor of Physics at Michigan State University, the National Superconducting Cyclotron Laboratory, and the Facility for Rare Isotope Beams, (FRIB) where she currently is Scientific Director.
Alexandra’s research is focused on the structure of short-lived rare isotopes using γ-ray spectroscopy and nuclear reactions. As Director of the FRIB she ensures that the scientific promise of the facility is achieved by an active user community.
She has served on the Nuclear Science Advisory Committee to the Department of Energy and the National Science Foundation, and on the writing group for the 2023 US Nuclear Science Long Range Plan. She has also played a major role in assembling whitepapers on rare isotope research and instrumentation for the FRIB era.
Alexandra is an author on more than 300 peer reviewed publications. She is a Fellow of the American Physical Society and the American Association for the Advancement of Science. Among other honors and awards she is the recipient of the Szymański Prize, a DOE Outstanding Junior Investigator award, and a Sloan Research Fellowship. Importantly, she has mentored many graduate students and postdoctoral research associates who moved on to productive careers in academia, national laboratories, and industry.
Alexandra earned her undergraduate and graduate degrees at the University of Koelm in Germany: Vordiplom in Physics, Diploma Thesis (on Experimental Nuclear Physics), and Dr. rer. nat (PhD equivalent in Physics).
CV: https://people.frib.msu.edu/~gade/cv.html
Webpage(s):
1. https://frib.msu.edu/about/organization/staff/gade-profile
2. https://people.frib.msu.edu/~gade/
3. https://frib.msu.edu/for-students/faculty/gade-profile
LinkedIn Profile
https://www.linkedin.com/in/alexandra-gade-65900424
Minutes
On October 18, 2024, Members of the Society and guests joined the speaker for a reception and dinner at 5:45 PM in the Members’ Dining Room at the Cosmos Club. Thereafter they joined other attendees in the Powell Auditorium for the lecture proceedings. In the Powell Auditorium of the Cosmos Club in Washington, D.C., President Larry Millstein called the lecture portion of the 2,503rd meeting of the Society to order at 8:07 p.m. ET. He began by welcoming attendees, thanking sponsors for their support and announcing new members. Scott Mathews then read the minutes of the previous meeting which included the lecture by Marc Okrand, titled “Klingon and Other Constructed Languages in the Real World”. The minutes were approved as read.
President Millstein then introduced the speaker for the evening, Alexandra Gade, of the Michigan State University Facility for Rare Isotope Beams. Her lecture was titled “The Science of Rare Isotopes”.
The speaker began by defining rare isotopes as those not found on Earth. She indicated that there are fewer than 300 stable isotopes, but there may be as many as 7000 possible isotopes. She discussed the processes by which unstable nuclei decay to stable nuclei: alpha decay, beta decay, gamma emission, positron emission, electron capture, and spontaneous fission. She then introduced the nuclear chart, often called the table of nuclides, which shows a graphical representation of all known nuclides plotted with the neutron number on the x-axis and the proton number on the y-axis. Using the table of nuclides, Gade described how an unstable nucleus can move toward regions of greater stability through the various decay processes. She described the proton dripline and neutron dripline, terms arising from the nuclear liquid drop model, as the limit of the number of protons or neutrons that can be bound to a nucleus. She indicated that the proton dripline, being governed by Coulomb repulsion, was well defined, but that the neutron dripline was largely unknown.
Gade then discussed the formation of rare isotopes on Earth, using the techniques of: light particle reactions, neutron reactions, fusion, and fragmentation/spallation. She went on to discuss the formation of rare isotopes in stellar environments, such as super novae and neutron star mergers. She described the competition between neutron capture, pushing nuclei towards the dripline, and beta decay, pushing nuclei back towards stability. Gade then discussed the techniques for producing rare isotopes in the laboratory, using particle beams. A stable nucleus is accelerated to about half the speed of light, and smashed into a target nucleus. The fragments from this collision are “fished-out and guided to experimental stations.” She described how magnetic fields are used to separate or filter the various isotopes by charge-to-mass ratio. She showed a brief video, graphically demonstrating each step in this process.
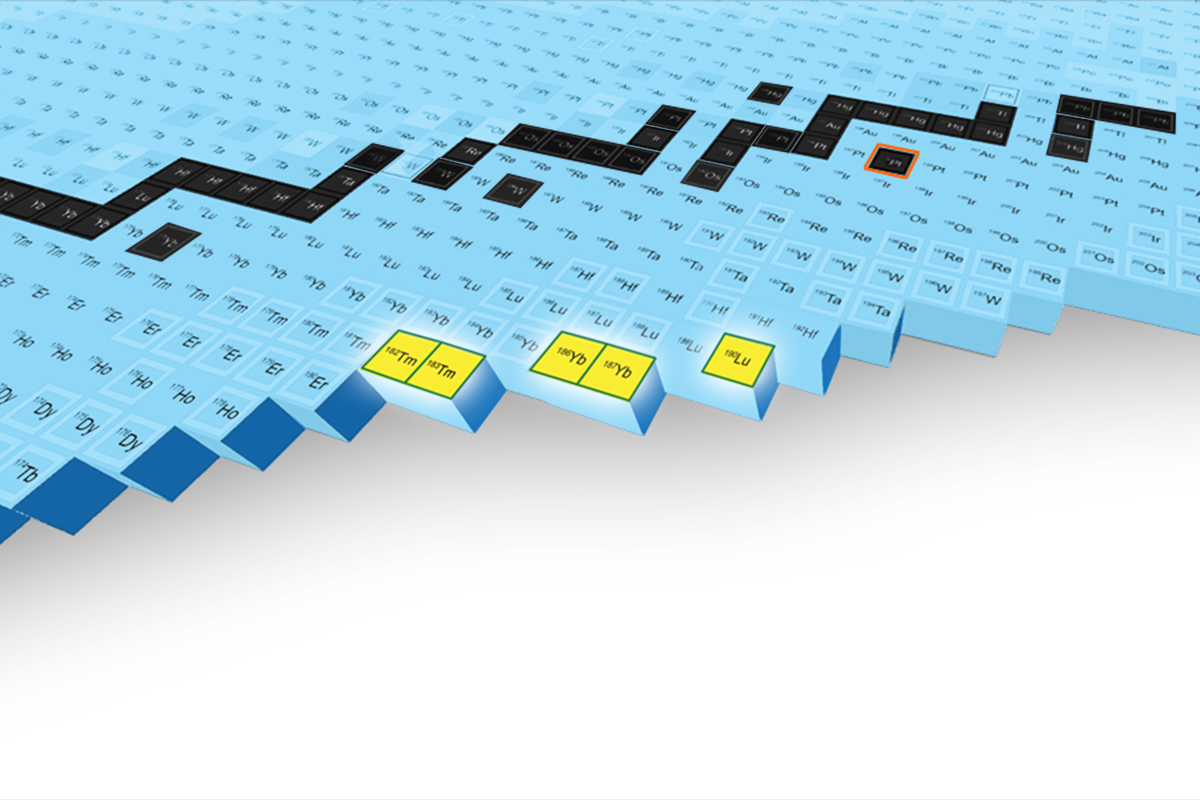
Gade then described the discovery of five new isotopes at her facility, called FRIB (Facility for Rare Isotope Beams), which included heavy isotopes of Thulium, Ytterbium, and Lutetium. This involved accelerating stable, Platinum-198 nuclei, on to various target nuclei, and separating and identifying the fragments to confirm the presence of these five, previously unobserved nuclei. She claimed that these isotopes are formed in neutron star mergers, and that understanding the properties of these isotopes is therefore crucial to understanding nucleosynthesis of heavy elements.
The speaker then discussed the formation of new elements, particularly the super-heavy elements. Although the FRIB beamline is not capable of producing such elements, she described the processes for synthesis, identification, and confirmation, indicating that many of the recently confirmed new elements have been synthesized in Russia and Japan. These super-heavy elements decay via alpha decay. Gade described how researchers follow the alpha decay chain in reverse: starting from a known daughter nucleus, counting the alpha decays, back up to the parent nucleus, in order to identify the new element. She said that it often takes years or even decades to officially recognize a new element, as it must be produced in multiple facilities, by multiple techniques, before it is accepted. Gade said that very recently, Lawrence Berkley Lab showed that super-heavy elements can be produced using a titanium beam, and that the US is now back in the race to produce new, super-heavy elements.
Gade then discussed the motivation for studying rare isotopes, including a variety of applications. She discussed how the nuclear physics of rare isotopes enables more accurate modelling of stellar events, like neutron star mergers. She discussed medical isotopes used for both diagnostic and therapeutic applications. She showed examples of rare isotopes being used to image real-time biological processes and to destroy cancer tumors. She discussed the role of rare isotopes in the design and modelling of nuclear batteries, particularly for long duration space probes, and the role of rare isotopes in modelling nuclear weapons in the absence of nuclear testing. She mentioned the use of rare isotopes for studying radiation-hardened electronics for space applications.
The speaker concluded her talk by saying that rare isotopes play an important role in fundamental physics and allow a variety of technological applications from biology to microelectronics.
The lecture was followed by a Question and Answer session:
A member asked about the use of rare isotopes to enhance our understanding of the nuclear force. Gade indicated that this was exactly the point of her research. She said she worked closely with theoreticians to validate and modify nuclear models, including models of the nuclear force.
A member asked about one of Gade’s charts, showing the rate of discovery of new isotopes as a function of time, citing the fact that there was a significant spike around 2010 or 2011. Gade responded that during that time period, a new facility for studying rare isotopes came online in Japan. This new facility discovered more than 100 new isotopes in its first two years of operation.
A member on the live stream asked if rare isotopes are responsible for a significant fraction of the radiation we see in the universe. Gade responded that while x-ray burst are the most frequent explosions in the universe, the vast majority of the observed radiation is not coming directly from rare isotopes, however, a significant fraction of the total power radiated is due to rare isotopes.
After the question and answer period, President Millstein thanked the speaker and presented her with a PSW rosette, a signed copy of the announcement of her talk, and a signed copy of Volume 1 of the PSW Bulletin. He then announced speakers of up-coming lectures, made a number of housekeeping announcements, and invited guests to join the Society. He adjourned the 2,503rd meeting of the society at 10:03 pm ET.
Temperature in Washington, DC: 14.4° Celsius
Weather: Fair
Audience in the Powell auditorium: 36
Viewers on the live stream: 28
For a total of 64 viewers
Views of the video in the first two weeks: 506
Respectfully submitted, Scott Mathews: Recording Secretary