COVID-19 Treatments and Trials
Treatments to End a Pandemic
Davey Smith
Professor of Medicine
University of California at San Diego
About the Lecture
This talk will start by describing the current understanding of SARS-CoV-2 pathogenesis and how it leads to COVID-19. It will then discuss issues with early studies on the treatment of COVID-19, using hydroxychloroquine as a case study. The presentation will next describe the current landscape of potential therapies, their targeted COVID-19 mechanisms, and how trials of these potential therapies are being conducted. The US government’s Operation Warp Speed program will be described with a focus on the ACTIV-2 trial, which aims to identify effective and safe treatments for people with COVID-19 that can keep them out of the hospital.
About the Speaker

Davey Smith is Vice-Chair of Faculty in the Department of Medicine, Chief of the Division of Infectious Diseases and Global Public Health and Professor of Medicine at UC-San Diego, and he also serves as Co-Director of the San Diego Center for AIDS Research and the Florence Seeley Riford Chair in AIDS Research there.
Davey is a translational research virologist. He uses basic science techniques to answer clinically relevant questions and vice-versa. He conducts research at both UC San Diego Antiviral Research Center and his laboratory on the UC San Diego campus. He been primarily involved in studying both the transmission of HIV and HIV reservoir dynamics to understand the factors that drive HIV transmission and persistence, and to find new ways to interrupt these mechanisms.
In late 2019, when reports of a novel coronavirus began circulating, Smith refocused much of his research on investigating causes, preventatives, and potential cures for the new virus, working together with colleagues in the Division of Infectious Diseases and Global Public Health at UC San Diego. In March 2020, he instigated the formation of collaborative partnerships across the 17 NIH-funded Centers for AIDS Research (CFARs) to pool laboratory equipment, methods, and knowledge applicable to HIV and easily transferable to studies of the novel coronavirus, helping to speed research on COVID-19. Currently, Davey leads or in involved in numerous COVID-19 studies across the United States.
Among other honors and awards, Davey was named HIV Researcher of the Year by the HIV Medical Association and was elected as a Fellow to the American Society of Clinical Investigation and of the American College of Physicians.
Davey earned his MD at the East Tennessee State University School of Medicine. He did his internship, residency and chief residency in Internal Medicine at UC San Diego, and stayed there to complete a fellowship in Infectious Diseases.
Minutes
On September 25, 2020, by Zoom videoconference broadcast on the PSW Science YouTube channel, President Larry Millstein called the 2,425th meeting of the Society to order at 8:02 p.m. EDT. He announced the order of business and welcomed new members. The Recording Secretary then read the minutes of the previous meeting.
President Millstein then introduced the speaker for the evening, David Smith, Professor of Medicine at the University of California at San Diego. His lecture was titled, “COVID-19 Treatments and Trials: Treatments to End a Pandemic.”
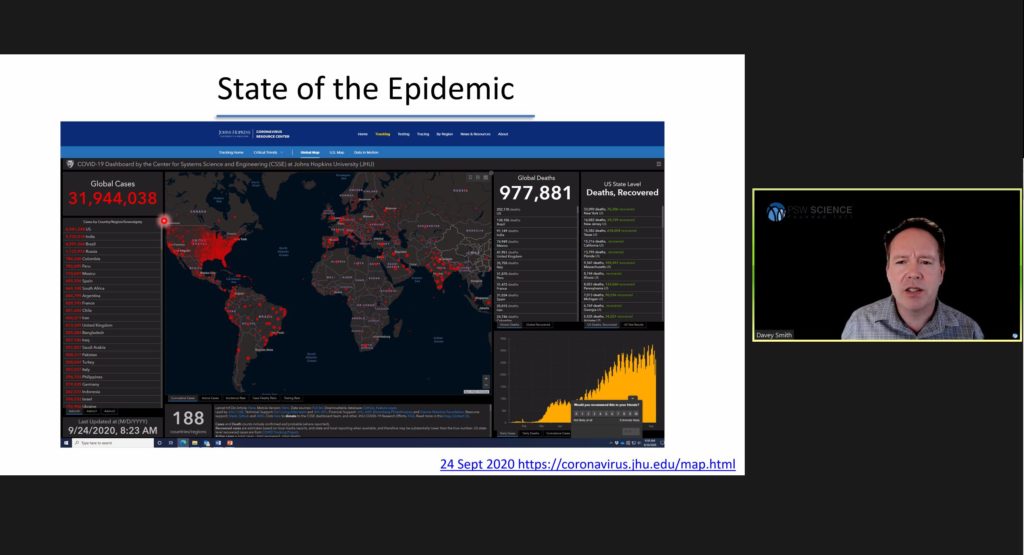
As of Smith’s lecture, there were approximately 32,000,000 million confirmed global SARS-CoV-2 /COVID-19 cases, and almost 1,000,000 million global deaths. The objective of Smith’s lecture was to answer the question, “Can we treat our way out of a pandemic?”
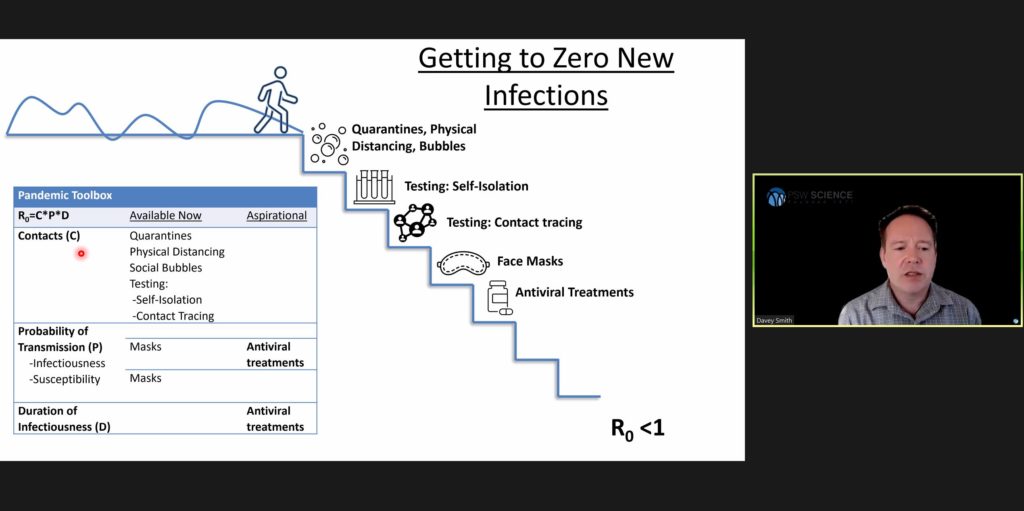
The reproductive rate of an infectious disease is calculated by multiplying the number of contacts an infectious person makes by the probability of transmission per contact and the duration the infected person is infectious to others. For SARS-CoV-2, the reproductive rate is between 3 and 5 secondary infections in a completely susceptible population.
Current efforts to reduce the number of new infections to zero are aimed at all three elements of the reproductive rate equation. Quarantines, physical distancing, and social bubbles, are intended to reduce the number of person-to-person contacts. Mask-wearing reduces the probability of transmission. Anti-viral treatments and vaccines are being developed to decrease both the probability of transmission and the duration of infectiousness.
Smith then specifically focused his lecture on the development of anti-viral treatments. Anti-viral treatments will be most effective against SARS-CoV-2 in the early stages of infection, when viral loads are highest while infected persons are showing mild to no symptoms. Initial treatment efforts began by researching whether existing drugs could be re-purposed to treat SARS-CoV-2. Examples include ACE inhibitors, Camostat, hydroxychloroquine, and Remdesivir.
In early 2020, data from cell cultures showed hydroxychloroquine to effectively inhibit viral growth. Early human tests produced similar results, and the FDA gave Emergency Use Authorization for hydroxychloroquine to be administered to infected persons showing symptoms. Before Smith and the National Institutes of Health could complete a trial on the drug, reports emerged that hydroxychloroquine was dangerous to patients and the FDA revoked its Emergency Use Authorization.
In retrospect, Smith said, because anti-viral treatment is most effective in early-stage SARS-CoV-2 infections, administering hydroxychloroquine in late-stage infections likely had minimal effect on the virus. A small-sample size study from the Henry Ford Foundation showed a higher survivability rate among people who received hydroxychloroquine early, compared to those who received a placebo.
Remdesivir was developed to treat SARS-CoV-1, called Middle Eastern Respiratory Syndrome (MERS), and has been repurposed to treat SARS-CoV-2. Gilead Pharmaceuticals owns the drug and conducted a trial that showed patients treated with remdesivir experienced a shorter recovery time than those given a placebo. Because remdesivir has a short half-life and must be administered intravenously, Smith said it has limited use outside hospital settings.
Developed to treat ebola, EIDD 2801 interferes and causes a virus to make fatal errors when attempting to duplicate its RNA. EIDD 2801 proved effected against MERS, and is now in clinical trials for use against SARS-CoV-2.
Scientists are also working on new drugs, including antibody therapies. Current efforts include collecting plasma from a recovered person and administering it to infected persons. Smith said there was a terminated, small sample trial which produced early data that convalescent plasma treatment could be effective in patients with severe, but not life-threatening, cases of SARS-CoV-2.
Smith and others are working on monoclonal antibodies. Some recovered people produce highly effective antibodies which can be identified, purified and expanded, and used to treat other infected persons. This strategy has been effectively implemented to treat ebola and HIV.
Another current approach by SAB Pharmaceuticals is to transplant a human immune system into cows, expose the cows to SARS-CoV-2 to prompt a polyclonal antibody response, which can be used for human treatment.
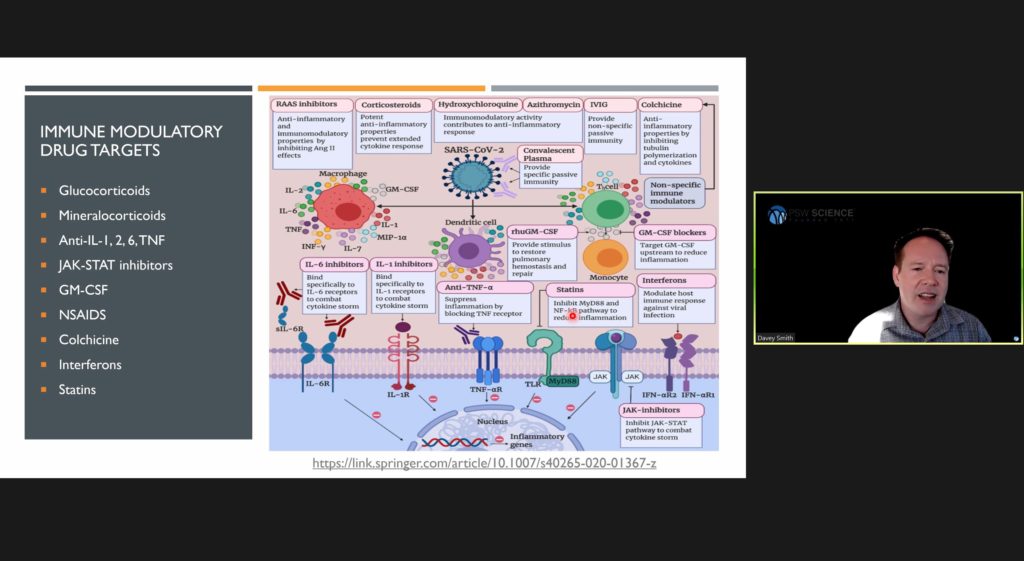
Smith said severe inflammation in persons infected with SARS-CoV-2 is caused by immune system overreaction. Immunomodulators and anticoagulants are being explored to treat such cases. Recent trials suggest higher survivability by hospitalized patients treated with low-dose Dexamethasone and steroids.
Smith is the Chair of the U.S. federal government’s Operation Warp Speed, or Active-2, to develop SARS-CoV-2 treatments as quickly as possible. Active-2’s objective is to rapidly and efficiently evaluate multiple potential therapeutics for COVID-19 in an outpatient setting to prevent them from requiring hospitalization. If the study determines an agent will prevent death or hospitalization through 28 days after study entry, the FDA will approve the drug.
Smith is optimistic and believes that yes, we can treat our way out of the SARS-CoV-2 pandemic.
The speaker then answered questions from the online viewing audience. One member asked about using colchicine to treat the virus. Smith said the drug will probably be used to treat late-stage infections, but is still under study. Another member asked about a small study on calcifidiol in Spain. Smith said the Spanish study was excellent, and that larger studies are needed. He said, but for the delay between the initial outbreak in China and the initiation of rigorous studies, many of the treatment questions would already be answered.
The robust question and answer period then continued with detailed questions and answers regarding various clinical trials and details about the virus.
After the question and answer period, President Millstein thanked the speaker, made the usual housekeeping announcements, and invited guests to join the Society. At 9:28 p.m., President Millstein adjourned the meeting.
Temperature in Washington, D.C.: 18° C
Weather: Showers
Concurrent Viewers of the Zoom and YouTube live stream, 86 and views on the PSW Science YouTube and Vimeo channels: 359.
Respectfully submitted,
James Heelan, Recording Secretary