The Presidents' Lecture
The Year Nanomaterials Saved the World from COVID-19
Nanoparticle-Enabled Delivery of Nucleic Acid Medicines
Michael J. Sailor
Distinguished Professor of Chemistry and Biochemistry
Director, Materials Research Science and Engineering Center
University of California at San Diego
Sponsored by Millen, White, Zelano & Branigan, PC
About the Lecture
With the recent approval of the first siRNA-derived therapeutic, and the deployment of the mRNA-based Moderna and Pfizer coronavirus vaccines, medicines that deploy nucleic acids as their active ingredient are undergoing a rapid transition from research to the clinical space. A primary enabler here has been the nanoscale vehicles used to deliver these agents effectively in vivo. This lecture will discuss the challenges in design and synthesis of nanoparticles for these applications: (1) protection of the nucleic acid payload from degradation and clearance; (2) selective homing to target cell types; (3) escaping or bypassing endocytic uptake to effect cytoplasmic release of the payload; and (4) minimizing toxicity to the host. The presentation will be given from the perspective of a nanomaterials scientist, with a focus on the emerging field of silicon-based nanoparticle systems.
References for further information
1. Kim, B.; Sun, S.; Varner, J. A.; Howell, S. B.; Ruoslahti, E.; Sailor, M. J., Securing the Payload, Finding the Cell, and Avoiding the Endosome: Peptide-Targeted, Fusogenic Porous Silicon Nanoparticles for Delivery of siRNA. Adv. Mater. 2019, 31 (35), 1902952.
2. Kim, B.; Park, J.-H.; Sailor, M. J., Rekindling RNAi Therapy: Materials Design Requirements for In Vivo siRNA Delivery. Adv. Mater. 2019, 31, 1903637.
3. Kim, B.; Pang, H.-B.; Kang, J.; Park, J.-H.; Ruoslahti, E.; Sailor, M. J., Immunogene therapy with fusogenic nanoparticles modulates macrophage response to Staphylococcus aureus. Nat. Commun. 2018, 9, 1969.
About the Speaker

Michael J. Sailor is Distinguished Professor, Chemistry & Biochemistry, Director of the Materials Research Science and Engineering Center and Co-Director of the Institute for Materials Discovery & Design at the University of California – San Diego. He also has affiliate appointments in the Bioengineering Department, the Nanoengineering Department, and the Materials Science and Engineering program at UCSD.
Michael’s research focuses on nanotechnology, with emphasis on biomaterials, drug delivery, imaging, and sensing applications. He is an expert in the chemistry, electrochemistry, and optical properties of nanomaterials, in particular porous silicon-based systems.
He is the author on more than 300 research publications, one book, and an inventor in 29 patents. He has founded three companies and served on the scientific advisory boards of six others.
Among other honors and awards Michael is an elected Fellow of the American Association for the Advancement of Science, the U.S. National Academy of Inventors, and the Royal Society of Chemistry.
Michael earned a BS at Harvey Mudd College and an MS and PhD at Northwestern University. He then did post-doctoral research at Stanford and CalTech.
Minutes
On January 8, 2021, by Zoom videoconference broadcast on the PSW Science YouTube channel, President Larry Millstein called the 2,432nd meeting of the Society to order at 8:02 p.m. EST. He announced the order of business and welcomed new members. The Recording Secretary then read the minutes of the previous meeting.
President Millstein then introduced the speaker for the evening, Michael J. Sailor, Distinguished Professor of Chemistry and Biochemistry, and Director of the Materials Research Science and Engineering Center at the University of California at San Diego. His lecture was titled, “The Year Nanomaterials Saved the World from COVID-19: Nanoparticle-Enabled Delivery of Nucleic Acid Medicines.”
The Covid-19 vaccines currently approved for use in the United States developed by Pfizer and Moderna are both enabled by nanotechnology. Both vaccines utilize messenger RNA (mRNA) enabled by a lipid nanoparticle delivery system. The forthcoming Novavax vaccine also is enabled by nanoparticles.
The anti-cancer drug Doxil was the first FDA-approved nano-drug. Approved in 1995, Doxil used nanoparticles to improve the solubility of doxorubicin and to mitigate its harsh side-effects by using the enhanced permeability and retention (EPR) effect to concentrate the drug in tumors rather than circulating throughout the body.
Doxil showed that nanoparticles could improve the efficacy of existing drugs, rescue “failed” drugs, and showed the promise of nanoparticles to protect emerging “biologic” therapeutics.
Unlike small molecule drugs, nucleic acids degrade quickly inside the body. To be effective, these therapies need to be protected until they can be delivered directly into the cell. To achieve these objectives, the Pfizer and Moderna Covid-19 vaccines both embed mRNA in a lipid nanoparticle and surround it in a lipid outer shell.
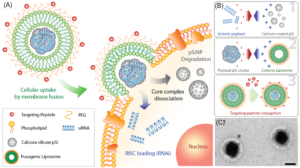
Sailor’s lab makes nanomaterials from silicon because the body readily excretes it without ill effect. Beginning with silicon wafers, Sailor’s lab uses electrochemical etching to produce free-standing porous silicon nanoparticles.
Unlike neutral molecules such as doxorubicin, DNA and RNA are highly charged. To coax them together, Sailor’s lab first used cationic lipids to ion pair with the nucleic acids to balance their charge. The lab subsequently used calcium chloride to “cement” nucleic acids into the pores of their silicon nanoparticles, achieving up to 20x higher mass loading compared to the conventional lipid-based nanoparticles.
Unprotected, nucleic acids “cemented” to nanoparticles would dissolve quickly in the human body. To solve that problem, Sailor’s lab “wrapped” the nanoparticles in a lipid coating. Sailor said these coated nanoparticles should fully release their payloads into the body over an approximately 24-hour period.
To target nanoparticle delivery of therapeutics directly into cells, Sailor’s lab coats the cationic head group of lipids around the nanoparticle with polyethylene glycol. This fusogenic coating causes the nanoparticle to fuse with the cell wall, rather than being consumed and assaulted by the cell’s defenses. The lipid coating then dissolves and the nucleic acids are released directly into the cell.
To target the desired cells, Sailor’s lab uses peptides with strong affinities for specific cell types to allow nanoparticles to selectively bind inside the body.
Sailor then described how his lab employed their techniques to deliver anti-inflammatory therapeutics to treat staph. aureus infections in mice. They cemented a siRNA payload of Irf5 genes to silicon nanoparticles, coated them in fusogenic liposomes, and added peptides to target macrophages that cause fatal inflammation. All control mice died, while all treated mice survived.
The speaker then answered questions from the online viewing audience.
One member asked whether there is a targeting peptide on the Covid-19 nanoparticles. Sailor said he understood those vaccines do not target specific cells.
Another member asked whether the nanoparticle treatment caused any damage to mice livers in the staph. aureus treatment experiment. Sailor said his team looked for and did not find any lesions on the livers of treated mice.
After the question and answer period, President Millstein thanked the speaker, made the usual housekeeping announcements, and invited guests to join the Society. At 9:40 p.m., President Millstein adjourned the meeting.
Temperature in Washington, D.C.: 2° C
Weather: Partly Cloudy
Concurrent Viewers of the Zoom and YouTube live stream, 88 and views on the PSW Science YouTube and Vimeo channels: 156.
Respectfully submitted,
James Heelan, Recording Secretary