Vaccines to Combat the Opioid Epidemic
Kim Janda
Professor of Chemistry
Director, Worm Institute of Research and Medicine
The Scripps Research Institute
About the Lecture
Substance abuse disorders are a global public health concern. In the United Sates opioid abuse is such an acute problem that Congress and the President recently declared a “National Emergency for Opioid Crisis”. The ability to prevent the spread of opioid abuse and to aid opioid addicts is severely handicapped by the lack of adequate prevention and treatment modalities. Indeed, many of those receiving the best treatments relapse nonetheless. There is an urgent need to discover effective medications to treat abuse of opioids and other substances.
Traditional, small-molecule approaches have been, at best, only marginally successful. “Biologics” based therapeutics offer a promising alternative to the customary small molecule approaches for treating abuse and reducing lethal effects of these drugs. This lecture will discuss how vaccination can alter the pharmacokinetic properties of opioids without untoward side effects. It will detail the chemistry, immunology and behavioral findings from the most recent vaccines against opioids that we have developed, including those against heroin, the fentanyls, oxy/hydrocodone and mitragynine.
About the Speaker

Kim Janda is the Callaway Professor in the Departments of Chemistry & Immunology and Microbial Science at The Scripps Research Institute, where he is also the Director of the Worm Institute of Research and Medicine.
Kim is well known for bridging the fields of chemistry and immunology. Early in his career, he showed how antibodies can be transformed from passive binding entities into dynamic chemical catalysts widely know as catalytic antibodies. In later work, he developed synthetic methods for creating and developing chemical and biochemical diversity libraries, including phage display and small molecule DNA encoding methods. He also developed an inexpensive diagnostic test for River blindness, based on his discovery of a small molecule neurotransmitter unique to the disease. More recently, Kim has been working on a new approach for treating drug addiction by harnessing the immune system to reject drugs of abuse in much the same ways it deals with agents of infectious disease.
He is an author on more than 600 peer-reviewed papers, and he has delivered more than 350 invited lectures.
He has received many awards for his work, including the American Chemical Society’s Alfred Bader Award in Bioinorganic or Bioorganic Chemistry,the Cope Scholars Award, and a Doctor of Philosophy honoris causa from the University of Helsinki, Finland. Kim is a AAAS Fellow. His work has been reported often in the media, including Discover Magazine, The New Yorker, The Economist, Wired, The New York Times, and numerous other newspapers and TV news outlets.
He earned a BS Clinical Chemistry at the University of South Florida and a PhD in Chemistry at the University of Arizona.
Minutes
On April 26, 2019, in the John Wesley Powell Auditorium of the Cosmos Club in Washington, D.C., President Larry Millstein called the 2,408th meeting of the Society to order at 8:02 p.m. He announced the order of business, that the evening’s lecture would be livestreamed on the internet, and welcomed new members to the Society.
President Millstein then introduced the speaker for the evening, Kim Janda, Professor of Chemistry at The Scripps Research Institute and Director of the Worm Institute of Research and Medicine. His lecture was titled, “Vaccines to Combat the Opioid Epidemic.”
Janda began with an overview of human vaccine use. He said the first use by by the Chinese in approximately 1000 CE, using variolation to prevent disease. The 19th century brought the first modern vaccine and is credited to Edward Jenner.
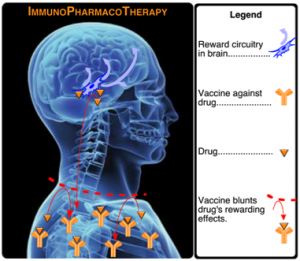
Janda said that unlike disease, the body must be taught to recognize a drug as a foreign object. The development of active vaccines is interdependent upon three critical aspects: hapten/linker design, a carrier protein, and adjuvants. Success in all three elements is required to produce an effective vaccine.
A hapten is a molecule that triggers an immune response. An effective vaccine hapten closely mirrors the unwanted disease or molecule. To vaccinate against a drug, the hapten must mirror the chemical structure, account for all chemical determinants, and be stable over time. To effectively elicit an immune response, the hapten must be “linked” to a carrier protein. Hapten coupling efficiency and copy number can greatly influence the immune response, making hapten/linker design crucial to developing an effective vaccine. Janda said the best protein linkers are usually 4-8 atoms in length, and position the hapten for optimal regiochemical display of chemical determinants. Upon vaccination, adjuvants such as alum and pathogen associated molecular patterns present antigens to the immune system.
When a drug vaccine is administered, it is taken up by dendtritic cells and displayed onto the major histocompatibility region allowing T helper cells to stimulate B cells, which then produce drug-neutralizing antibodies. In other words, scientists are attempting to create antibodies (which do not cross the blood/brain barrier) to prevent certain drugs from reaching the brain. If the antibody has enough affinity to the drug, Janda said the antibody may be able to “pull back” the drug from the brain.
Janda then discussed his work developing vaccines against opioids. Janda said that vaccines are superior to these current treatment methods for opioid addiction because vaccines do not require continual compliance, should reduce risk of overdose, and avoid adverse side effects.
Janda then addressed heroin, a semi-synthetic opioid which crosses the blood/brain barrier and there quickly reduces to 6-acetylmorphine, and then to morphine. To properly vaccinate against heroin, Janda hypothesized that the most effective vaccine would be dynamic, using a hapten that mirrors heroin and then slowly degrades to allow the body to create antibodies to heroin and to its two degraded forms.
Janda tested his hypothesis by comparing a dynamic heroin vaccine to a combination of static vaccines for heroin, 6-acetylmorphine, and morphine. Using rodent hotplate and tail flick tests, Janda concluded the dynamic heroin vaccine was more effective than combining the three static vaccines.
Janda also used animal self-administration tests to evaluate the dynamic heroin vaccine. These tests showed the heroin vaccine blocked the re-escalation of compulsive heroin seeking behavior, and reduced motivation to take heroin following re-escalation.
Janda then described his work in improving vaccine stability. Wheras traditional vaccines must be stored and transported cold, Janda’s heroin vaccine has remained efficacious after 30 days of storage at room temperature. The vaccine can also be lyophilized into a powder and reconstituted.
Janda has adapted the process they used for the heroin vaccine to a vaccine for prescription opioids hydrocodone and oxycodone, which have similar chemical structures and normally have a half-life of 20-30 minutes. Janda’s team was unable to produce a vaccine that differentiated between the two drugs. However, the vaccine was able to create immune response that created antibodies effective for both drugs and increased their half-life to approximately 200 hours.
Janda then addressed synthetic opioids. Synthetic opioids, like fentanyl, quickly transfer from blood plasma to receptor sites in the brain. These drugs are stored in fat tissue, requiring lengthy treatment of short half-life overdose drugs like naloxone to be effective.
Janda said his fentanyl vaccine has proven more effective than his heroin vaccine, has shown effective for treating overdose, and is effective against carfentanil and other fentanyl-related drugs. Tested in mice, Janda’s fentanyl vaccine was faster and more effective in treating overdose than naloxone. He is optimistic that rather than leaving an overdose patient on an extended overdose drug treatment, they could be administered the fentanyl vaccine and sent home. Janda then described the process of creating humanized monoclonal antibodies using transgenic rats, and said his fentanyl vaccine is now ready for testing in non-human primates.
Future drug treatment work will need to address development in the drug market. For example, there are increasing instances of heroin contaminated fentanyl. This development will require combination vaccines.
Further, vaccines are not a successful solution for all drugs. Nevertheless, Janda said is optimistic that scientists will find other solutions for drug addiction. For example, while Janda and others have tried and failed to vaccinate for nicotine, scientists have identified an enzyme solution to degrade nicotine that will help treat nicotine addition and acute nicotine poisoning.
One member asked how patients who experience withdrawal and are prone to use opioids again will be affected by opioid vaccines. Janda said vaccines will be useful to prevent relapse in addicts who desire to stop using drugs.
A guest asked how long it will take opioid vaccines to reach the market. Janda said that he is working with the National Institute on Drug Abuse to first bring human trials of monoclonal antibodies to carfentanil.
After the question and answer period, President Millstein thanked the speaker, made the usual housekeeping announcements, and invited guests to join the Society. At 9:37 p.m., President Millstein adjourned the meeting to the social hour.
Temperature: 22.5°C
Weather: Rainy
Attendance in the Powell Auditorium:
Viewing Through the Live Stream on the PSW Science YouTube Channel:
Respectfully submitted,
James Heelan, Recording Secretary



